Gas Absorption at Liquid Surface
| Introduction | Solve the PDE Model |
| Domain | Post-processing and Visualization |
| Mesh Generation | Nomenclature |
| Mass Transport Model | References |
| Boundary Conditions |
Introduction
Physical and chemical gas absorption are an important separation processes and widely employed in various industries. Gas absorption is used to either separate undesirable components from a gas or for manufacturing purposes of chemicals.
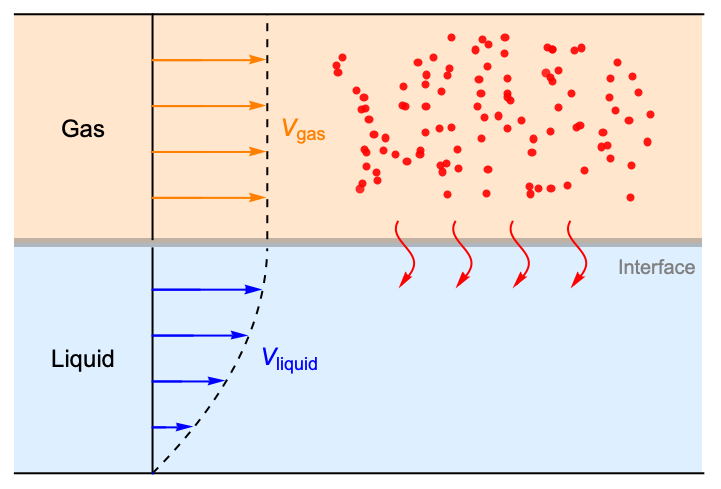
This example reproduces a gas absorption model [1]. The absorption process takes place in the gas-liquid interface section shown below in gray:
A gas is exposed to a fully developed laminar flow. The gas flows either co-currently or counter-currently with respect to the flowing liquid. The above image displays the co-current case. The solute in the gas flow is absorbed and removed by the liquid flow beneath it, and mass transfer of the solute takes place from the gaseous to the liquid phase. The time of contact between solute and liquid is long enough to presume a parabolic velocity profile. Steady state conditions are assumed to prevail. In the post-processing section we will compare the absorption effectiveness between the co-current case and the counter-current case.
Domain
To model the interphase mass transfer, we will define a thin interphase region between the gas and liquid flow, which will allow us to enforce an equilibrium condition (1) specified below via coupled fictitious mass sources and handle the discontinuous concentration of the solute between two different phases.
Here we set the thickness ![]() of the interphase region to be
of the interphase region to be ![]() of the height of the domain. Note that if
of the height of the domain. Note that if ![]() is set too small numerical instability may occur around the interface.
is set too small numerical instability may occur around the interface.
Mesh Generation
We start be generating a boundary ElementMesh that accounts for the different material regions and the interface region. On the boundary we specify markers that will be used later to set up boundary conditions for to co-current and counter-current case.
To accurately model the mass transfer between the gas and liquid phases, the interphase region should be finely meshed. For clarity we add the material regions as an Association.
Mass Transport Model
A separate mass balance equation is used to describe the species concentration in the gas and liquid phases:
Here ![]() and
and ![]() are the concentration of solute dissolved in the gas phase and liquid phase. To model the mass transfer between the two phases we add coupling mass source terms
are the concentration of solute dissolved in the gas phase and liquid phase. To model the mass transfer between the two phases we add coupling mass source terms ![]() and
and ![]() in the governing mass balance equation (2), leading to:
in the governing mass balance equation (2), leading to:
The above equations pose a system of 2 equations with each 3 material regions.
The diffusion coefficients of the solute in the gas and in the liquid phases are given by ![]() and
and ![]() respectively. Note that
respectively. Note that ![]() and
and ![]() are only active within the interphase region as well as their own phase.
are only active within the interphase region as well as their own phase.
Next, we specify the fluid flow velocities ![]() . The flow velocities are only active in their respective sub-regions. The gaseous flow velocity
. The flow velocities are only active in their respective sub-regions. The gaseous flow velocity ![]() will initially be in the same direction as the liquid flow velocity
will initially be in the same direction as the liquid flow velocity ![]() .
.
To model the mass transfer between two phases we add the coupling mass source terms ![]() and
and ![]() in the governing mass balance equation (3), leading to:
in the governing mass balance equation (3), leading to:
Here ![]() and
and ![]() are set at
are set at ![]() so that the equilibrium condition (4):
so that the equilibrium condition (4): ![]() can be enforced at the interface.
can be enforced at the interface.
In the equation ![]() is the interphase mass transfer coefficient, and
is the interphase mass transfer coefficient, and ![]() is a switch that turns on
is a switch that turns on ![]() within the interphase region and off
within the interphase region and off ![]() otherwise.
otherwise.
Based on the two-resistance theory, the equilibrium at the interface is considered to be reached instantaneously and maintained at all times. This condition can be modeled by setting the mass transfer coefficient ![]() to be infinitely large. In practice, we can choose
to be infinitely large. In practice, we can choose ![]() to be greater than the species diffusivity
to be greater than the species diffusivity ![]() and
and ![]() ) by several orders of magnitude.
) by several orders of magnitude.
At the interface of two phases the concentration ![]() and
and ![]() may be discontinuous. The ratio
may be discontinuous. The ratio ![]() is known as the equilibrium distribution coefficient
is known as the equilibrium distribution coefficient ![]() .
.
The coefficient ![]() depends on pressure, temperature and the chemical properties of the transported species and the media of both phases. The value
depends on pressure, temperature and the chemical properties of the transported species and the media of both phases. The value ![]() can be determined by experimental measurement [2]. In this example the equilibrium coefficient is given by
can be determined by experimental measurement [2]. In this example the equilibrium coefficient is given by ![]() .
.
Due to mass conservation, the coupling source terms ![]() and
and ![]() have the same magnitude but opposite sign.
have the same magnitude but opposite sign.
Boundary Conditions
The concentration of solute is set at ![]() at the gas inlet, and
at the gas inlet, and ![]() at the liquid inlet.
at the liquid inlet.
Solve the PDE Model
Post-processing and Visualization
To measure the effectivity of the gas absorption, the net reduction of the solute is calculated between the gas inlet and outlet. For this the mean concentration at the outlet is computed with a boundary integration:
In the co-current case, the solute in the gas flow has been reduced by ![]() .
.
Next we compare the result with the case of counter-current gas and liquid flow.
In the counter-current case, the solute in the gas flow has been reduced by ![]() , which is more effective than the co-current case.
, which is more effective than the co-current case.
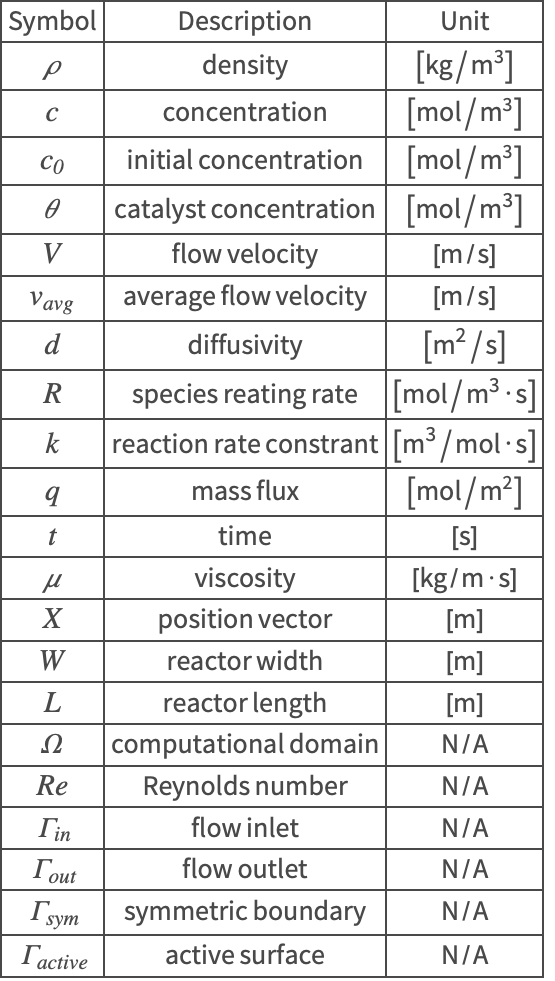
Nomenclature
References
1. Danish, M., Sharma, R.K. and Ali, S., Gas absorption with first order chemical reaction in a laminar falling film over a reacting solid wall, Applied Mathematical Modeling: 32 901–929 (2008).
2. Prausnitz, J. M., Lichtenthaler R. N. and de Azevedo, E. G. Molecular Thermodynamics of Fluid Phase Equilibria 3rd Ed., Prentice Hall PTR, New Jersey (1999).